Bridging the Gap: The CDC’s Bridge Access Program
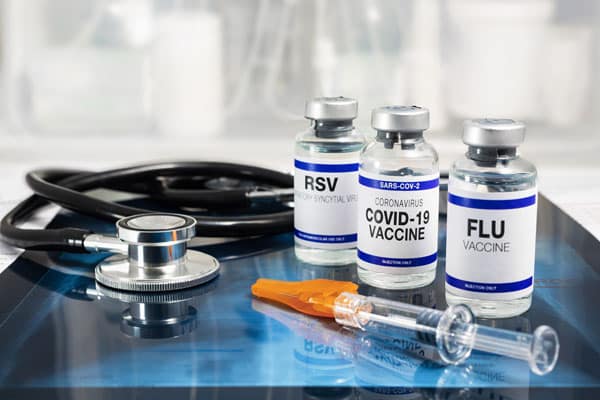
The US and CDC has taken a giant leap forward in ensuring all individuals have access to the 2023-24 COVID-19 vaccine by launching the Bridge Access Program. The Bridge Access Program offers a lifeline to adults without health insurance and those whose insurance doesn't cover the total cost of COVID-19 vaccines.